Abstract
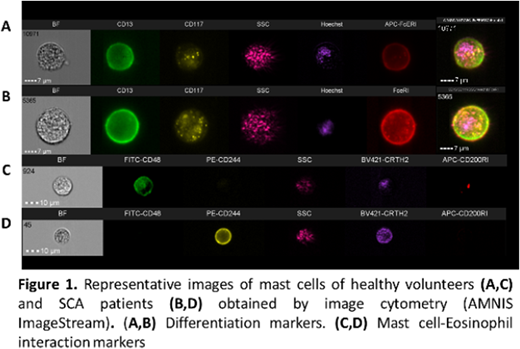
Introduction: Sickle cell anemia (SCA) is characterized by a chronic inflammatory state as a result of cellular activation and biochemical mediator release. Mast cell (MC) activation syndrome is recognized as a factor in the morbidity of SCA patients (Afrin 2014); in SCA murine models, MC activation and MC extracellular trap formation (Vincent et al, 2013; Tran et al 2017) are associated with neurogenic inflammation and nociceptor activation. Allergic models showed that MC activation induces selective release of preformed or newly synthesized compounds; these dynamics exhibit features that could be associated with distinct patterns of MC-dependent inflammation (Gaudenzo et al, 2016), which is modulated by the engagement of additional surface receptors activated by signals from other cells. In these models, MC and eosinophils (Eos) coexist in inflamed tissues and bidirectional modulation occurs; in SCA, however, the role of both types of cells has not been fully determined. Methods and Results: In order to clarify the contribution of MC/Eos cross talk to the pathophysiology of SCA, we standardized in vitro models to study MC that were differentiated from CD34+ stem cells and freshly isolated Eos from SCA patients and healthy volunteers (HV). First, using FACS and image cytometry (ImageStream-X; Amnis Corporation), we characterized surface marker expression (adhesion, activation and MC-Eos receptors, Fig 1) and identified changes on expression patterns after short-term MC/Eos co-cultures. HV-MC and SCA-MC were obtained after 5 weeks; higher frequencies of CD117+ and CD63+ cells were found on SCA-MC cultures but no other differences for MC or Eos were detected by FACS. Differences in cell size and the expression pattern distributions of CD117, CD63 and CD200RI between HV-MC and SCA-MC were found by IC analysis (Ratio Discrimination (RD) >1). Incubation of HV-MC and SCA-Eos for 2 hours generated changes not only in CD63 (RD 1.1) and CD117 (RD 1.3), but also in CD48 marker pattern expression (RD 1.09). The effect was more evident in SCA-Eos/SCA-MC cocultures for CD48 (RD 2.3) and CD63 (RD 2.2). Secondly, we compared the ability of SCA-MC and HV-MC to induce functional responses on Eos by evaluating changes in the frequency of apoptotic and activated (non-circular) Eos (IC analysis: Nuclear Morphology and Circularity Morphology Features). After 24 hours of MC/Eos coculture, the ability of MC and MC culture supernatants to induce Eos chemotaxis was evaluated by trans-well assays. After 24 hours, the percentage of apoptosis of Eos in monoculture was low (HV-Eos 1.2±0.3; SCA-Eos 3.65±1.1). MC/Eos coculture decreased the frequency of apoptotic Eos; where the anti-apoptotic effect on SCA-Eos was higher (4.2 fold) than that of SCA-MC (2-fold). Eos monocultures showed lower frequencies of activated cells (% of Eos with Circularity Morphology Index <10: HV-Eos 9.1±3.4, SCA-Eos 13.0±6.5); MC/Eos cocultures of both SCA-MC and HV-MC increased (2-3.5 fold) the percentage of activated SCA-Eos in comparison with Eos monocultures. SCA-MC strongly increased activated SCA-Eos (3.5-fold) and HV-Eos (2.4-fold) (p<0.05, 95% CI 5.5-43.69). The greatest chemotactic effect was obtained using SCA-MC supernatant stimuli for both HV-Eos (396.0%±53.8) and SCA-Eos (300.2%±22.5) in comparison with basal (100%) and eotaxin-1 (100 ng/ml) controls (HV-Eos 257.8%±76.4; SCA-Eos 227.7%±12.1; Eos p=0.020; MC p=0.006; Interaction p=0.61). Finally, we studied the frequency of heterotypical (MC-Eos) conjugate formation by IC analysis and we measured the influence of Eos on MC degranulation dynamics using a colorimetric test (β-hexosaminidase, β-hex). MC/Eos conjugate formation was a rare event (less than 5% of images); there was no difference in the percentage of conjugates among MC types (data not shown). Degranulation of SCA-MC was characterized by higher β-hex activity (HV-MC 0.03±0.009; SCA-MC 0.34±0.11 Units/ml). β-hex activity was reduced when incubated with HV-Eos (0.19±0.12 Units/ml) and SCA-Eos (HV-MC 0.04±0.010 Units/ml). Conclusions: Our data indicated that mast cells and eosinophils interact to modulate the activities (cell activation, degranulation, apoptosis) of each other and can contribute to the perpetuation of the inflammatory response in SCA. These bidirectional interactions between mast cells and eosinophils might be important to the pathophysiology of SCA. Financial Support: FAPESP.
Fertrin:Apopharma Inc.: Honoraria; Alexion Pharmaceuticals Brasil: Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal